Preserve QOL
KISQALI helps preserve your patients’ quality of life. *(1-4)
KISQALI + ET consistently preserved QOL across phase III trials and achieved a time to deterioration (TTD) ≥10% across trials. *†‡(1-4)
Patient reported outcomes for health-related QOL were exploratory secondary endpoints. QOL was assessed using the EORTC QLQ-C30 questionnaire: a validated tool used worldwide to assess QOL in patients with cancer. (1,3–5)
* MONALEESA-2: HR 0.94; 95% CI: 0.72-1.24; MONALEESA-3: HR 0.81; 95% CI: 0.62-1.06; MONALEESA-7: HR 0.67; 95% CI: 0.52-0.86. (1–4)
† TTD was defined as ≥10% worsening of the scales scores relative to baseline, with no later improvement above this threshold observed during the treatment period, or death due to any cause, in the global health status/QOL score. (1,3,4)
‡ The most common adverse events across the pooled MONALEESA studies with a reported frequency ≥20% were neutropenia, infections, nausea, fatigue, diarrhoea, leukopenia, vomiting, headache, constipation, alopecia, cough, rash, back pain, anaemia and abnormal liver function tests.(6)
MONALEESA-2 study results
Overall change from baseline in patient-reported EORTC QLQ-C30 global health status/QOL(1)
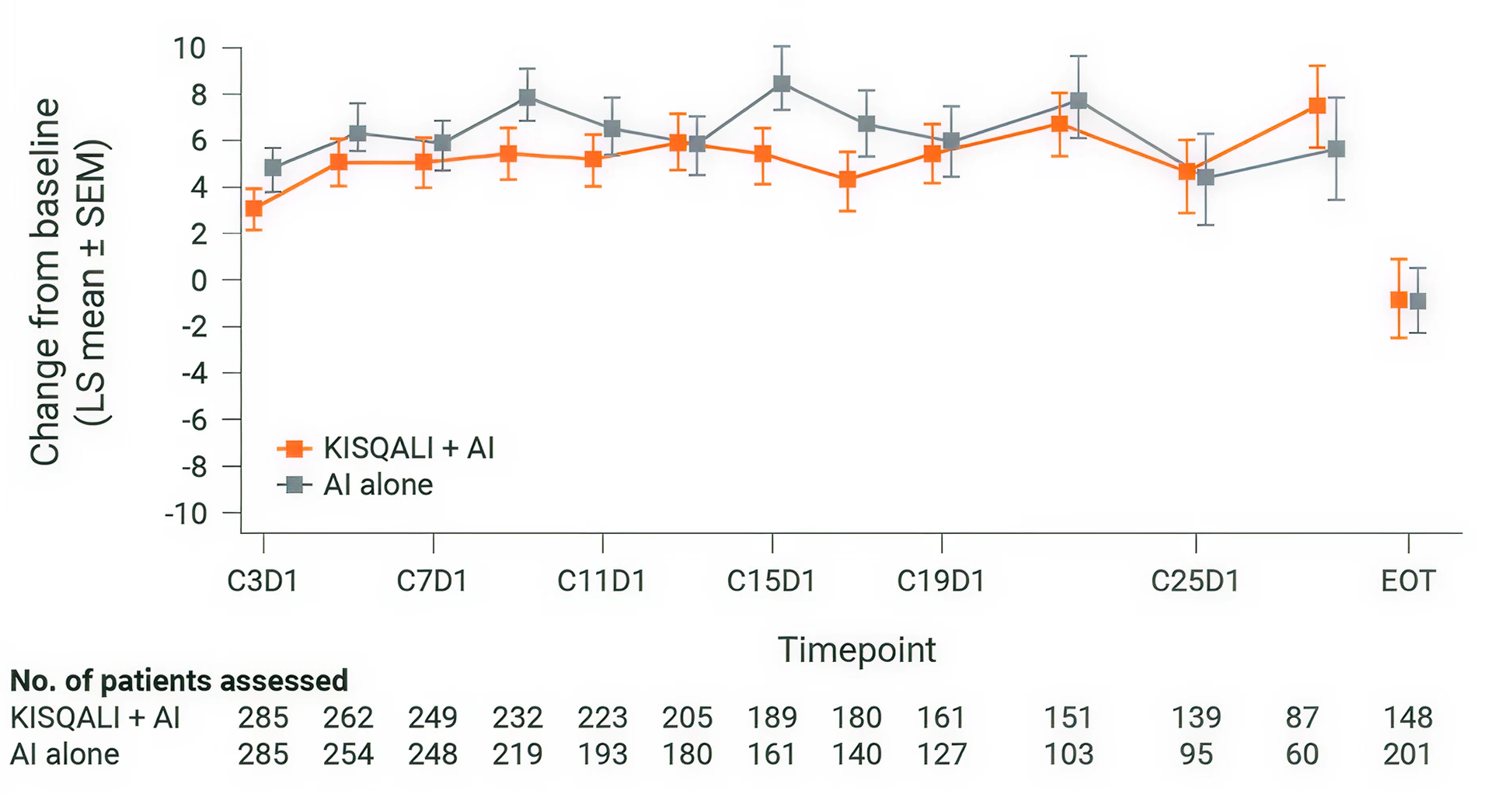
KISQALI + AI maintained QOL (mTTD ≥10%) for 27.7 months vs 27.6 months with AI alone (HR 0.94; 95% CI: 0.72-1.24). (1,2)
Adapted from Verma S, et al., 2018.
MONALEESA-2: N=668, double-blind, placebo-controlled, 1:1 randomised, multicentre, phase III trial in postmenopausal women with HR+/HER2– ABC. As 1L in advanced disease. No prior endocrine therapy for ABC and no previous systemic chemotherapy for advanced disease. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + AI (letrozole 2.5 mg continuous). The primary endpoint was locally assessed PFS, and the key secondary endpoint was OS. Other secondary endpoints included the ORR (complete or partial response), the CBR (overall response plus stable disease lasting 24 weeks or more), safety, and QOL assessments. (5)
MONALEESA-3 study results
Overall change from baseline in patient-reported EORTC QLQ-C30 global health status/QOL(3)
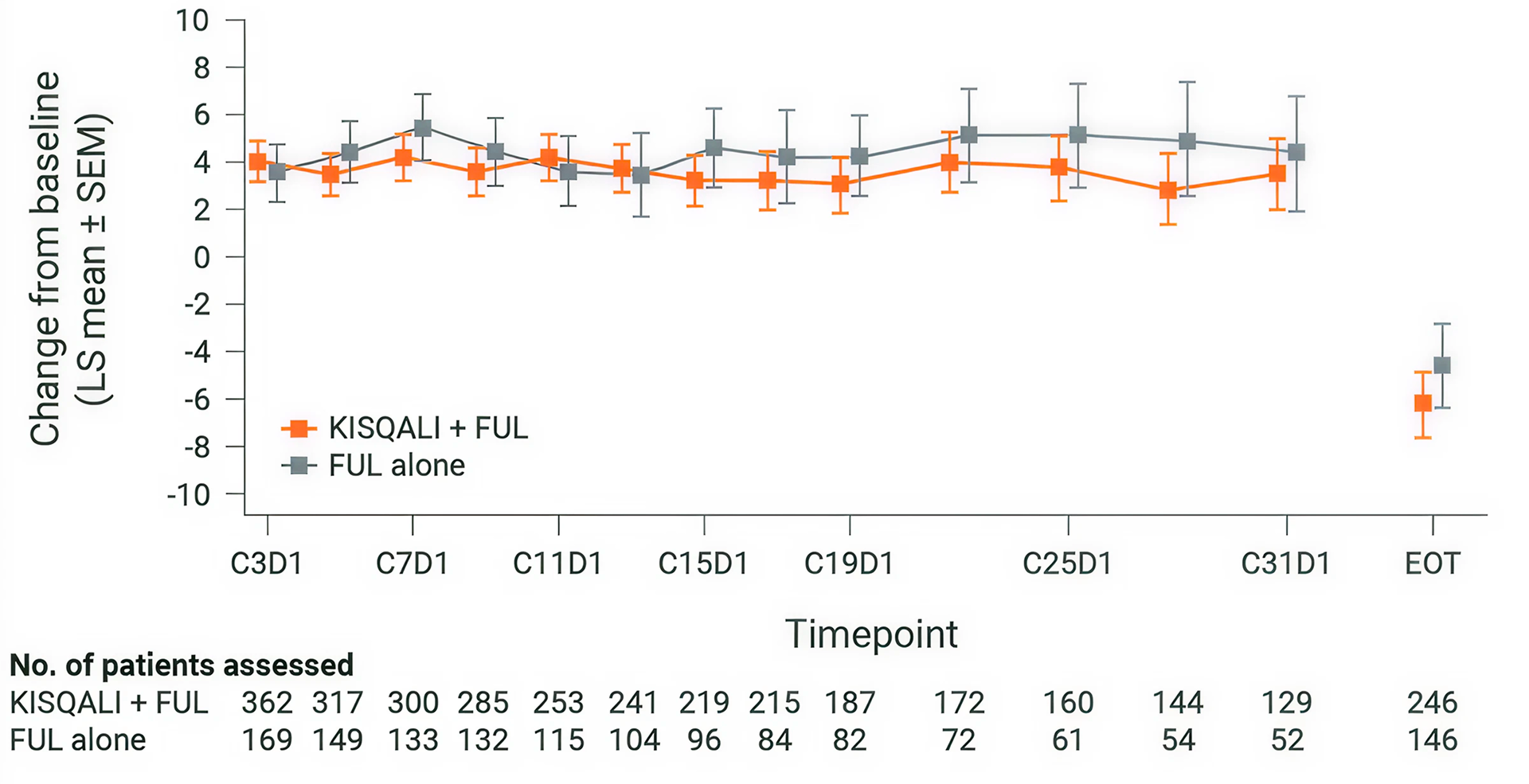
KISQALI + fulvestrant maintained QOL (mTTD ≥10%) for 35.9 months vs 33.1 months with fulvestrant alone (HR 0.81; 95% CI: 0.62-1.06). (3)
Adapted from Fashing PA, et al., 2020.
MONALEESA-3: N=726, double-blind, placebo-controlled, 2:1 randomised, phase III trial. As 1L and 2L in advanced disease plus those with early relapse in postmenopausal women with HR+/HER2– ABC. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + 500 mg intramuscular fulvestrant. The primary endpoint was locally assessed PFS. Secondary endpoints included OS, ORR, CBR, and safety and tolerability.
1L defined as: newly diagnosed (de novo) ABC patients or patients with relapse >12 months from completion of (neo)adjuvant ET with no treatment for ABC or metastatic disease. 2L was defined as: relapse on or within 12 months from completion of (neo)adjuvant endocrine therapy with no treatment for advanced or metastatic disease (early relapse); relapse >12 months from completion of (neo)adjuvant therapy with subsequent progression after one line of endocrine therapy for advanced or metastatic disease, and advanced or metastatic breast cancer at diagnosis that progressed after one line of endocrine therapy for advanced disease with no prior (neo)adjuvant treatment for early disease. (7)
MONALEESA-7 study results
Overall change from baseline in patient-reported EORTC QLQ-C30 global health status/QOL(8)
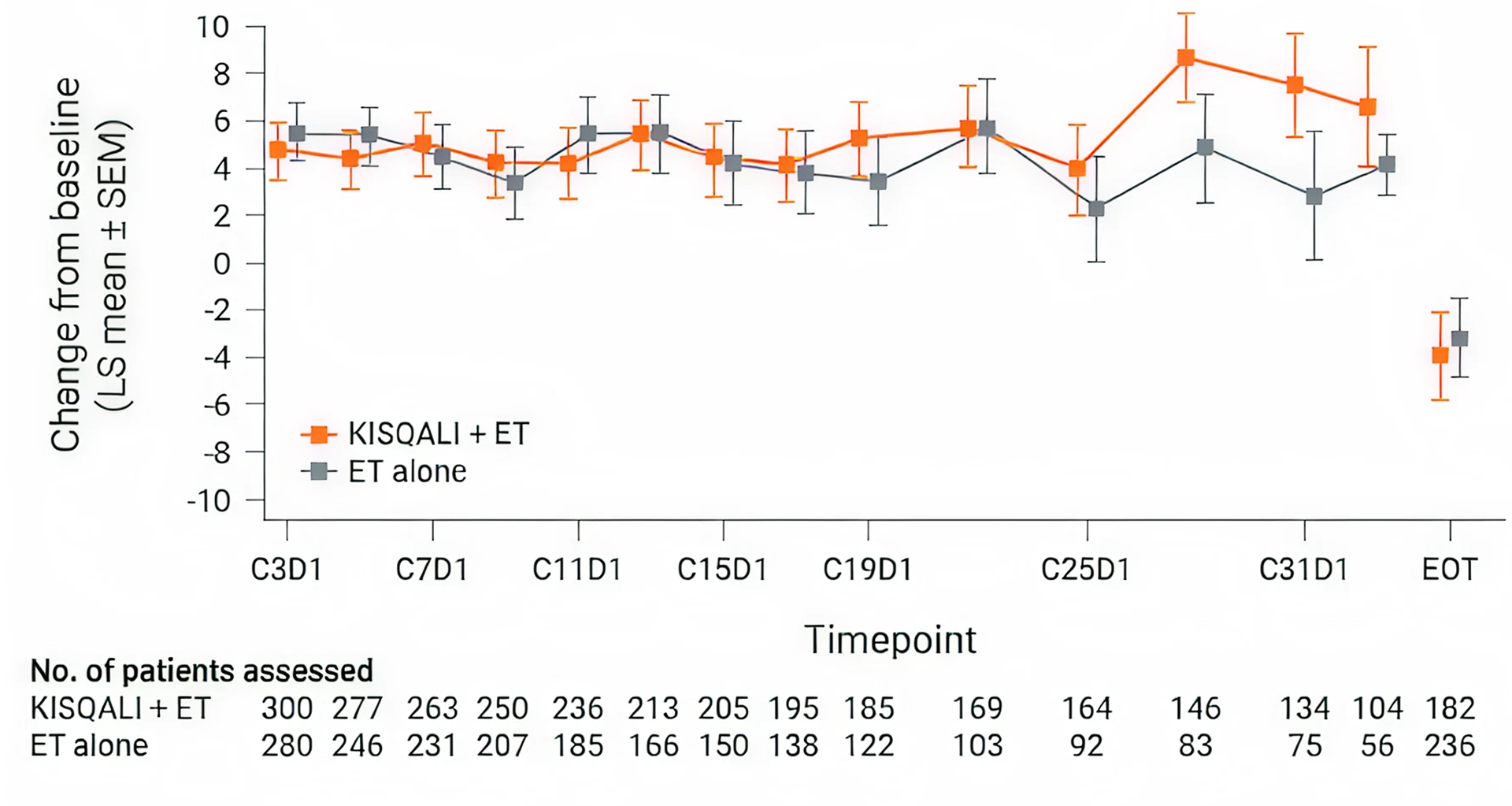
KISQALI + ET maintained QOL (mTTD ≥10%) for 35.8 months vs 23.3 months with ET alone (HR 0.67; 95% CI: 0.52-0.86). (4)
Adapted from Harbeck M, et al., 2020.
MONALEESA-7: N=672, double-blind, placebo-controlled, 1:1 randomised, phase III trial in pre- or perimenopausal women with HR+/HER2− ABC. As 1L in advanced disease and in patients who received 1 or fewer lines of chemotherapy for ABC. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + AI (letrozole 2.5 mg or anastrozole 1 mg) or tamoxifen* 20 mg orally once daily continuously + LHRH agonist (goserelin 3.6 mg subcutaneously on Day 1 of every cycle). The primary endpoint was investigator-assessed PFS. The key secondary endpoint was OS, defined as the time from randomisation to death from any cause. (9)
* KISQALI should not be co-administered with tamoxifen. (6)
QOL vs abemaciclib
MAIC of patient-reported symptoms. (1)
This was an anchored MAIC comparing symptoms with KISQALI vs abemaciclib, both in combination with AI. Individual patient data from MONALEESA-2 and published aggregated data from MONARCH-3 were included in this analysis. The analysis was performed using patient-reported outcome data from the EORTC QLQ-C30 and BR-23.
Matching-adjusted indirect comparisons are used in the absence of head-to-head trials, there are currently no head-to-head trials between KISQALI and abemaciclib. The MONALEESA-2 and MONARCH-3 datasets are not directly comparable due to differences in study populations, designs, and treatment arms, including placebo groups. Adjustments were made for the comparison, however, the results have limitations and should be interpreted with caution.
The TTSD analysis significantly favoured KISQALI + AI in four symptom scales vs abemaciclib + AI. *(1)
← Slide to view more →
BR23, breast cancer-specific quality of life questionnaire; QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire
* Notably, the TTSD analysis did not significantly favour abemaciclib + AI over KISQALI + AI in any functional or symptom scale of the QLQ-C30 or BR-23 but did numerically trend in favour of abemaciclib + AI in financial difficulties (HR 1.13; 95% CI: 0.60–2.14) and breast symptoms (HR 1.26; 95% CI: 0.62–2.55). (1)
† Including pain in arm or shoulder, swollen arm or hand, and raising arm. (1)
MONALEESA-2: N=668, double-blind, placebo-controlled, 1:1 randomised, multicentre, phase III trial in postmenopausal women with HR+/HER2− ABC. As 1L in advanced disease. No prior ET for ABC and no previous systemic chemotherapy for advanced disease. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + AI (letrozole 2.5 mg continuous). The primary endpoint was locally assessed PFS, and the key secondary endpoint was OS. Other secondary endpoints included the ORR (complete or partial response), the CBR (overall response plus stable disease lasting 24 weeks or more), safety, and QOL assessments.(2)
MONARCH-3: N=493, double-blind, placebo-controlled, 2:1 randomised, multicentre, phase III trial in postmenopausal women with HR+/HER2− ABC. As 1L in advanced disease. No prior endocrine therapy for advanced disease and no previous systemic chemotherapy for advanced disease. Abemaciclib 150 mg or placebo orally twice daily, continuously, plus AI (anastrozole 1 mg or letrozole 2.5 mg continuous). The primary endpoint was investigator-assessed PFS, and the key secondary endpoint was OS. Other secondary endpoints included ORR (percentage of patients with best response of complete or partial response), duration of response (time from complete or partial response until disease progression or death), CBR (percentage of patients with best response of complete response, partial response, or stable disease ≥ 6 months), and safety and tolerability.(3)
QOL vs combination chemotherapy
Improvement in QOL with 1L KISQALI + ET vs combo CT in patients with aggressive disease. *(1)
1L KISQALI + ET is associated with better symptom-related QOL vs combo CT. (1)
* Aggressive disease features included symptomatic visceral metastases, rapid disease progression or impending visceral compromise, or markedly symptomatic non-visceral disease. (2)
† mTTD based on the composite endpoint for overall health status score. (1)
‡ mTTD based on composite endpoint of TOI score – a scale of overall physical and functional well-being as well as breast cancer symptoms. (1)
(§) HR 0.63; 95% CI: 0.44–0.90. (1)
(II) HR 0.59; 95% CI: 0.42–0.85. (1)